The Right Track for Vision Correction
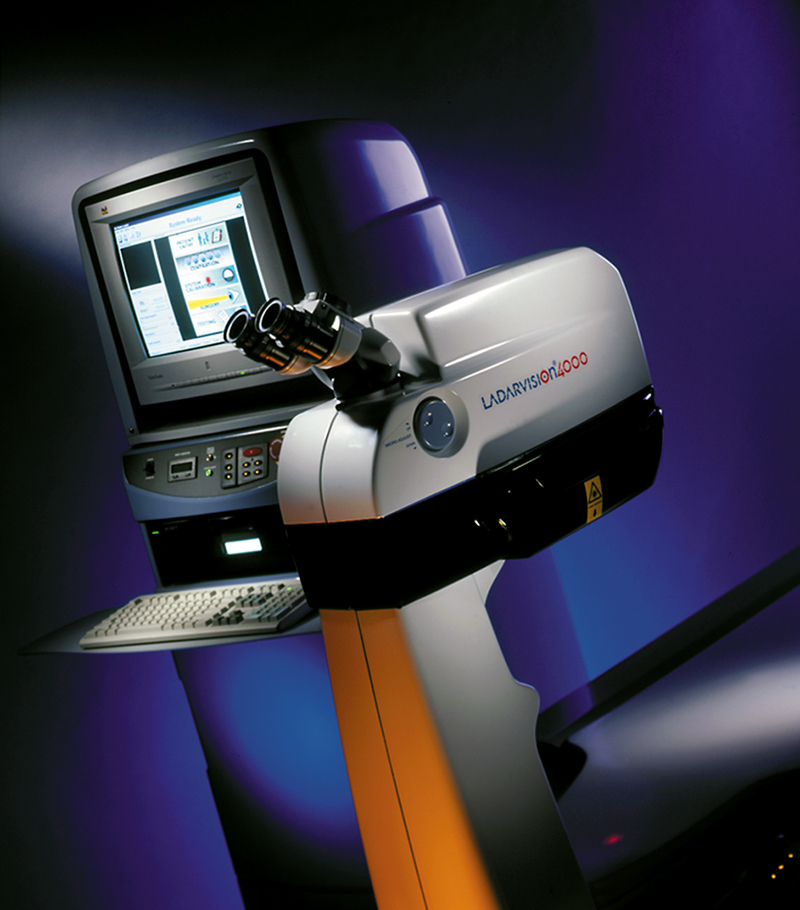
More and more people are putting away their eye-glasses and contact lenses as a result of laser vision correction surgery. LASIK, the most widely performed version of this surgical procedure, improves vision by reshaping the cornea, the clear front surface of the eye, using an excimer laser. One excimer laser system, Alcon's LADARVision® 4000, utilizes a laser radar (LADAR) eye tracking device that gives it unmatched precision.
During LASIK surgery, laser pulses must be accurately placed to reshape the cornea. A challenge to this procedure is the patient's constant eye movement. A person's eyes make small, involuntary movements known as saccadic movements about 100 times per second. Since the saccadic movements will not stop during LASIK surgery, most excimer laser systems use an eye tracking device that measures the movements and guides the placement of the laser beam.
The eye tracking device must be able to sample the eye's position at a rate of at least 1,000 times per second to keep up with the saccadic movements. Eye tracking devices vary greatly in speed depending upon their type. The most commonly used video tracking systems follow eye movements between 60 to 250 times per second, meaning that they cannot keep up with saccadic movements. Therefore, when the eye moves too far from the limits set by the eye surgeon, the surgeon must shut down the laser beam and restart the surgery once the laser is properly centered.
Sufficient speed is not a problem for Alcon's patented LADARTracker,™ a LADAR eye tracking device that measures eye movements at a rate of 4,000 times per second, 4 times the perceived safety margin. The LADARTracker also employs a closed-loop system, which keeps the device locked on the eye at all times. Eye movement information is continuously relayed to the system, allowing the system to compensate for the movements. Video tracking systems, on the other hand, are open-loop systems that try to follow eye movements rather than compensate for them. LADARVision is currently the only excimer laser device that continually monitors and tracks eye movement through the closed-loop system.
LADARVision's eye tracking device stems from the LADAR technology originally developed through several Small Business Innovation Research (SBIR) contracts with NASA's Johnson Space Center and the U.S. Department of Defense's Ballistic Missile Defense Office (BMDO). In the 1980s, Johnson awarded Autonomous Technologies Corporation a Phase I SBIR contract to develop technology for autonomous rendezvous and docking of space vehicles to service satellites. During Phase II of the Johnson SBIR contract, Autonomous Technologies developed a prototype range and velocity imaging LADAR to demonstrate technology that could be used for this purpose. LADAR was also used in military and NASA-sponsored research for applications in strategic target tracking and weapons firing control.
Autonomous Technologies' work for NASA in the area of pointing and scanning laser beams aided the development of the eye tracking device and the LADARVision system. With its advances in LADAR, Autonomous Technologies decided to enter the excimer laser business to develop a system for refractive surgery. In 1998, the U.S. Food and Drug Administration (FDA) granted Autonomous Technologies approval to market its LADARVision system for the correction of nearsightedness, farsightedness, and astigmatism. Shortly afterwards, a subsidiary of Summit Technology, Inc., an industry leader in ophthalmic excimer laser technology, merged with Autonomous Technologies, forming Summit Autonomous.
Alcon acquired Summit Autonomous and the LADARVision technology in May 2000, placing the Fort Worth, Texas-based company at the forefront of refractive surgical technology. The company's LADARVision 4000 made another breakthrough by combining the benefits of the LADAR tracking device with a flying, small-spot laser beam. This small, narrow laser beam is 0.8 millimeters wide, permitting a very precise, gradual corneal shaping. The eye surgeon can closely calibrate the beam to remove the proper amount of corneal tissue for correction of the refractive errors that cause vision problems. The system has the only FDA-approved claim of improved accuracy in corneal shaping.
Eye surgeons across the country are utilizing the LADARVision 4000 for LASIK surgery. In October 2002, Alcon's LADARVision system, consisting of the LADARVision 4000 and the LADARWave wavefront measurement device, became the first to gain FDA approval for wavefront-guided LASIK. The resulting procedure, called CustomCornea, allows surgeons to measure and address visual distortions that previously went undetected. The precision of the tracking device and the small spot beam make the LADARVision system the premier equipment to deliver these precise treatments.
LADARVision® is a registered trademark of Alcon.
LADARTracker™ is a trademark of Alcon.
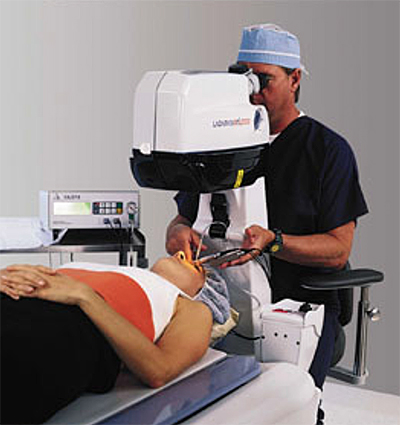
During LASIK surgery, Alcon’s LADARTracker™ measures eye movements at a rate of 4,000 times per second. This is 4 times the perceived safety margin for an eye tracking device.

LADARVision® is approved by the U.S. Food and Drug Administration for the correction of nearsightedness, farsightedness, and astigmatism through LASIK surgery.