Corrosive Gas Restores Artwork, Promises Myriad Applications
Originating Technology/NASA Contribution
Short wavelength solar radiation in the space environment just outside of the Earth’s atmosphere produces atomic oxygen. This gas reacts with spacecraft polymers, causing gradual oxidative thinning of the protective layers of orbiting objects, like satellites and the International Space Station, which maintain low-Earth orbit directly in the area where the corrosive gas is most present.
To combat this destructive gas, NASA engineers developed long-duration coatings that are resistant to the effects of its problematic presence. To validate the effectiveness of the coatings, NASA had two options: Either send the materials into orbit for testing, which would involve the cost of launches and severely limit access to the experiments, or recreate the atmospheric conditions here on Earth. NASA chose the latter, and the Electro-Physics Branch at Glenn Research Center constructed ground facilities to test the durability of different materials by introducing them to a recreated form of the corrosive space gas.
The experiments were successful, and the coatings are currently used on the International Space Station. In the experimentation, though, the scientists discovered several additional interesting applications for their test facilities and beneficial uses for atomic oxygen here on Earth.
Partnership
Led by Glenn’s Bruce Banks and Sharon Rutledge, the Electro-Physics researchers became familiar with atomic oxygen’s unique characteristic of oxidizing hydrogen, carbon, and hydrocarbon polymers at surface levels. While destructive to spacecraft polymers constructed with those materials, atomic oxygen’s selectivity could, they realized, also be applied in instances where someone wanted just those elements removed. Over the past few years since they made this realization, Banks and his team have partnered with several churches and museums to restore fire-damaged or vandalized artworks, and with an international forensics organization to develop new methods for detecting forged documents, as well as having developed a method for using atomic oxygen to remove bacterial contaminants from surgical implants.
Product Outcome
Atomic oxygen is able to remove organic compounds high in carbon (mostly soot) from fire-damaged artworks without causing a shift in the paint color. It was first tested on oil paintings. In 1989, an arson fire at St. Alban Episcopal Church, in Cleveland, nearly destroyed a painting of Mary Magdalene. Although the paint was blistered and charred, after 230 hours of atomic oxygen treatment and a reapplication of varnish, it was once again recognizable as a work of art. In 2002, a fire at St. Stanislaus Church, again in Cleveland, left two paintings with soot damage that the atomic oxygen process was able to remove.
Buoyed by the successes with oil paints, the team also applied the restoration technique to acrylics, watercolors, and ink. As long as the paints were primarily synthetic, the results were promising. They discovered though, that some organic acrylics and ink, in particular, required less exposure so that the atomic oxygen would not begin to wear away at the medium itself. This potential liability has been used advantageously, however, in instances of graffiti removal. Experiments showed that, by using a pencil-thin beam of atomic oxygen, the team was able to remove most inks except black permanent marker.
At Pittsburgh’s Carnegie Museum of Art, where an Andy Warhol painting, “Bathtub,” was kissed by a lipstick-wearing vandal, the technique successfully removed the offending pink mark with a portable atomic oxygen gun. The process lightened a spot of paint, but a conservator was easily able to match the spot, thus restoring the painting.
The successes with the art restoration process were well-publicized, and Lynda Taylor-Hartwick of the Independent Association of Questioned Document Examiners Inc. (IAQDE), a multinational, nonprofit professional organization dedicated to the art of forensic analysis of documents, read about the effects of atomic oxygen on ink and became curious about possible applications for this process in the field of forgery detection. She found that it can assist document analyzers in determining if, for example, checks or wills have been altered.
Atomic oxygen oxidation of ink may cause altered pen marks to look differently than the original marks. It can help examiners discriminate between two different inks, because different inks may oxidize at different rates, showing document examiners any signs of tampering. Usefulness, however, is not limited to instances where the inks are of different manufacture. Atomic oxygen, which oxidizes and removes organic materials by converting them into gasses, works gradually. Thus, thick layers of carbon or organic materials take longer to remove than thin layers. The ends of pen strokes tend to have much thicker ink deposits than the rest of the line, enabling the use of atomic oxygen exposure to determine which lines were drawn first, which strokes were made as one fluid movement, and which overlapped strokes have been added at a later date, a clear indication that a document has been altered.
The most telling sign, though, is the layering of ink that occurs when someone writes over a letter or number to alter it. Take, for example, the classic case of modifying a report card to turn an F into a B before showing the parents. To complete this feat, the belatedly concerned student would connect the lines at the top of the F with a curved stroke, making it more similar to the letter P, and then finish the job by looping in the base, thus raising the grade to a B. In order to make the job look good, though, the strokes must connect to the original letter, even overlap a little to make it look uniform. It is the overlapping, a miniscule amount of layering, that atomic oxygen can erode in order to expose the alteration.
While most parents may not go the extent of acquiring a portable atomic oxygen gun to check a report card, the application becomes more relevant for applications like determining check fraud or altered wills. Just as an F can become a B, a 1 can become a 9 or a 3 can become an 8, which could have potentially significant financial implications in instances of fraud.
It is not just paint and ink that the Glenn team is experimenting on, though. The gas has biomedical applications as well. Atomic oxygen technology can be used to decontaminate orthopedic surgical hip and knee implants prior to surgery. As a result of handling, fabrication, and exposure to air, the surfaces of these implants are often contaminated with endotoxins (naturally occurring compounds found within bacteria) and other biologically active contaminants. Such contaminants contribute to inflammation, which can lead to joint loosening, pain, and even the necessity to remove the implant. Previously, there was no known chemical process which fully removed these inflammatory endotoxins without damaging the implants. Atomic oxygen, however, can oxidize endotoxins and any other organic contaminants to convert them into harmless gasses, leaving a contaminant-free surface.
The inventors have patented this application for atomic oxygen and believe it could lead to significant reduction in health care costs for the more than 2.8 million people who receive orthopedic implants annually. They also believe that it promises increased functional life of implants, as well as a reduction of inflammation and the associated joint pain that patients experience.
Additional collaborative research between the Cleveland Clinic Foundation and the Glenn team into the terrestrial uses of atomic oxygen shows that this gas’s roughening of surfaces even improves cell adhesion, which is important for the development of new drugs.
While this application is still in its testing stages, the others are available for use. The patent for atomic oxygen art restoration is now in the public domain. Use of the technology for document alteration detection was never patented, and it, too, is available in the public domain. A patent was licensed for the removal of biologically active components from surgical implants, and Glenn is currently in talks with a company that sells plasma treating equipment.
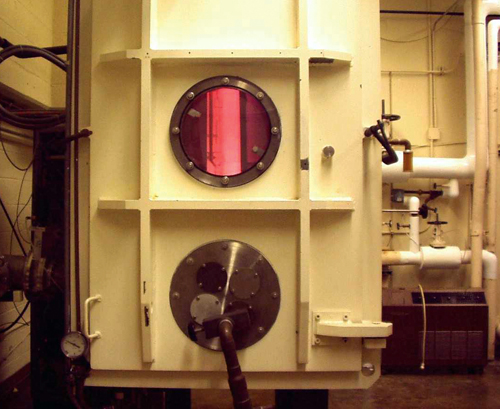
The Atomic Oxygen Exposure Facility in operation at Glenn Research Center has been used for the removal of smoke damage and aged varnish from the surface of paintings and for cleaning organic contaminants from surfaces of materials.

Parishioners at St. Alban Church, in Cleveland, thought this painting of Mary Magdalene was ruined after an arson fire destroyed much of the property. The same corrosive attributes of atomic oxygen that eat away at spacecraft were able to remove the layers of soot and smoke that covered the painting.