Circulation-Enhancing Device Improves CPR
Originating Technology/NASA Contribution
Ever stand up too quickly from a sitting or lying position and feel dizzy or disoriented for a brief moment? The downward push of Earth’s gravity naturally causes blood to settle in the lower areas of the human body, and occasionally, with a quick movement—such as rising swiftly from a chair—the body is not able to adjust fast enough to deliver an adequate supply of blood to the upper parts of the body and the brain. This sudden, temporary drop in blood pressure is what causes brief feelings of lightheadedness upon standing. In essence, when the heart pumps blood to different parts of the body, it is working against the physical phenomenon of gravity in its efforts to send blood up to the brain.
In more cases than not, the body is able to make the necessary adjustments to ensure proper blood flow and pressure to the brain; but when the disorientation lasts a long time and/or become chronic, individuals may have a condition called orthostatic intolerance. According to the American Journal of Physiology–Heart and Circulatory Physiology, an estimated 500,000 Americans are affected by orthostatic intolerance. Symptoms range from occasional fainting, blurry vision, and pain or discomfort in the head and the neck, to tiredness, weakness, and a lack of concentration. Though research indicates that the condition is not life-threatening, it could impact the quality of life and contribute to falls that result in serious injuries.
The condition is a prominent concern for NASA, since astronauts have to readjust to the gravitational environment of Earth after spending days in the weightlessness of space. NASA’s Exploration Systems Mission Directorate has found that roughly 20 percent of astronauts coming off of short-duration space flights experience difficulty maintaining proper blood pressure when moving from lying down to either sitting or standing during the first few days back on Earth. The difficulties are even more severe for astronauts coming off of long-duration missions, according to the mission directorate, as 83 percent of these crewmembers experience some degree of the condition.
Cardiovascular experts at NASA have found that the blood that normally settles in the lower regions of the body is instead pulled to the upper body in the microgravity environment of space. Blood volume is subsequently reduced as some cardiovascular reflexes are no longer being used, and less blood flows to the legs. Additionally, the muscles weaken, especially in the lower portion of the body, because they are not working (contracting) as hard as they usually do. This is not so much a concern for the astronauts while they are in space, since the action of floating around takes the place of putting center-of-gravity pressure on their legs. (They do exercise strenuously while in microgravity, though, to keep their muscles and circulatory systems conditioned, thus preparing their bodies for the return to gravity as best they can.) When they return to Earth’s gravity, however, more blood returns to the legs. Since there is a lower volume of blood, the flow that is supposed to be traveling to the brain can be insufficient. That is when orthostatic intolerance can set in.
NASA has conducted and sponsored a wealth of studies to counter the effects of orthostatic intolerance, especially since the condition could prevent an astronaut from exiting a landed spacecraft in the event of an emergency. In one study conducted by Johnson Space Center’s Cardiovascular Laboratory, astronauts in orbit tested the efficacy of a drug called midodrine that has successfully reduced orthostatic intolerance in patients on Earth. The early results were promising, but further testing will be conducted by the laboratory before more conclusive results can be determined. In another study, the laboratory is using a controlled tilt test on Earth to replicate the body’s responses to a shift from reclining to sitting or standing.
At Ames Research Center, researchers are utilizing NASA’s 20-G artificial gravity centrifuge machine in a pilot study on cardiovascular responses and fluid shifts in the body. A separate Ames study is evaluating the possibility of expanding astronauts’ plasma volumes (the fluid part of the blood, minus the blood cells), as a preventative measure.
In NASA-sponsored research at Vanderbilt University, researchers have successfully identified a genetic cause for orthostatic intolerance. The findings marked the first time a genetic defect had been linked to a disorder of the autonomic immune system, according to the discoverers, and could eventually lead to new drugs and treatments for the condition.
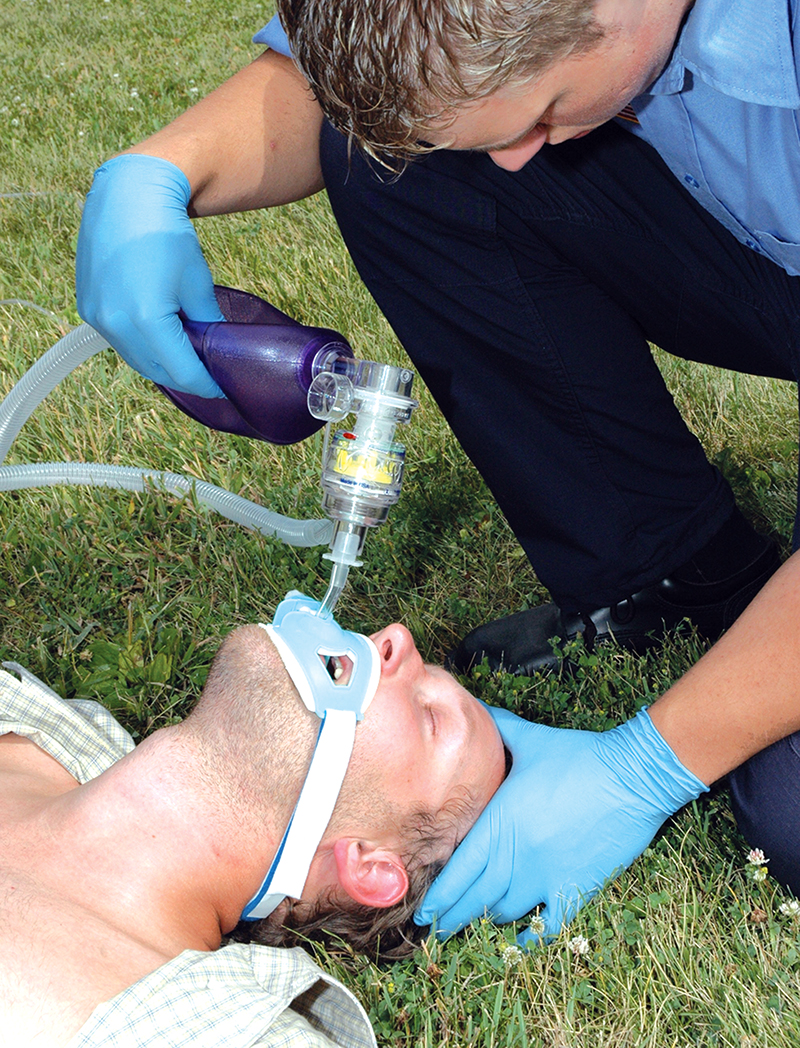
At Kennedy Space Center, a collaborative research effort with the U.S. Army and private industry has yielded an important application for a new, non-invasive medical device called ResQPOD that is now available for astronauts returning from space. In helping to reacquaint the astronauts with the feeling of gravity, ResQPOD quickly and effectively increases the circulation of blood flow to the brain. This device is also available to the public as a means to enhance circulation for breathing patients suffering from orthostatic intolerance and for non-breathing patients suffering cardiac arrest or other high-risk clinical conditions attributed to low blood pressure.
Partnership
Advanced Circulatory Systems Inc., of Minneapolis, collaborated with Kennedy and the U.S. Army Institute of Surgical Research for more than 5 years to develop ResQPOD. Don Doerr, an engineer at Kennedy, led the testing and development effort; Dr. Victor Convertino of the Institute of Surgical Research (and a former NASA scientist at Kennedy) also played an instrumental role in developing the technology.
Multiple clinical studies were conducted during the research effort, including six published studies. The published works demonstrate that ResQPOD offers a significant improvement in cardiac output and blood flow to the brain and in preventing shock in the event of considerable blood loss, when compared to conventional resuscitation. According to Advanced Circulatory Systems, data from the NASA studies played a major role in the company obtaining U.S. Food and Drug Administration 501K clearance for the device.
Dr. Keith Lurie, chief medical officer at Advanced Circulatory Systems and a primary member of the collaborative research effort, said, “The three-way partnership between NASA, private industry, and the U.S. Army Institute of Surgical Research is really a model for how organizations can work together to benefit both government programs and civilians.”
In 2006, Dr. Smith Johnston, the lead flight surgeon for NASA’s space shuttle missions, added ResQPOD to the list of medical equipment that is available for returning astronaut crews. The device was on hand for the landing of Space Shuttle Atlantis (STS-115) on September 21, 2006.
“We’re excited that our devices were available to the medical team [for the STS-115 mission] and look forward to continued collaboration with NASA to assist its efforts to safeguard the health of the astronauts,” added Lurie.
Product Outcome
Manufactured commercially by Advanced Circulatory Systems and distributed by Sylmar, California-based Tri-anim Health Services Inc., the ResQPOD circulatory enhancer improves upon the standard of care for patients with a variety of clinical conditions associated with low blood flow. Advanced Circulatory Systems’ primary commercial focus, though, is on non-breathing patients who can benefit from enhanced circulation, such as those experiencing cardiac arrest.
According to the American Heart Association, about 900 Americans fall victim to sudden cardiac arrest every day, with approximately 95 percent dying before they reach the hospital. This is why cardiopulmonary resuscitation (CPR) can mean the difference between life and death, as increasing blood flow to the heart and brain until the heart can be restarted is critical to improving survival rates with normal neurological functioning.
ResQPOD is an American Heart Association-rated Class IIa impedance threshold device, meaning that it is the highest recommended “adjunct” in the association’s latest guidelines for CPR. As a Class IIa impedance threshold device, it also carries a higher recommendation than any medication used to boost circulation in adults suffering cardiac arrest, according to these guidelines.
The device is about the size of a fist and can be affixed to either a facemask or an endotracheal breathing tube during CPR. It enhances the intrathoracic vacuum that forms in the chest during the chest recoil phase of CPR by temporarily sealing off the airway between breaths and preventing unnecessary air from entering the chest (timing-assist lights on the device will aid the rescuer in ventilating the patient at a proper rate). The vacuum that is created pulls blood back to the heart, doubling the amount of blood that is pulled back by conventional mouth-to-mouth/chest compression CPR, according to clinical studies, which also show that blood flow to the brain is increased by 50 percent. In sustaining proper blood flow to the heart and to the brain, ResQPOD increases the likelihood of survival and decreases the likelihood of neurological disorders.
ResQPOD is being used by emergency medical technicians in cities all around the country, including Boston, Houston, Indianapolis, Miami, and Oklahoma City, as well as Hartford, Connecticut; Kansas City, Missouri; Raleigh, North Carolina; and Toledo, Ohio. In some cities, it has reportedly increased the number of cardiac arrest patients delivered alive to the hospital by as much
as 50 percent. At Cypress Creek Emergency Medical Services (EMS), a large medical care organization serving more than 400,000 residents in the greater Houston area, ResQPOD has become a standard of care. Overall resuscitation rates climbed to nearly 50 percent since the organization began deploying the device in 2005, boosting hospital admission rates from 26 percent to an astounding 38 percent.
“These results are gratifying, and we applaud the entire Cypress Creek EMS organization for their advanced emergency medical service care and their ability to turn around the dismal statistics that surround cardiac arrest,” noted Advanced Circulatory Systems’ Lurie.
In its secondary commercial applications, Advanced Circulatory Systems is offering ResQPOD to improve circulation in patients suffering from orthostatic intolerance and general low blood pressure. These secondary uses also apply to individuals who undergo dialysis treatments and may experience a drop in blood pressure, as well as those who go into shock after severe blood loss.
Outside of the traditional hospital setting, the company is investigating the beneficial impact ResQPOD could have on wounded soldiers in the battlefield who may have lost a great deal of blood and are in danger of going into shock.
Advanced Circulatory Systems is also harnessing the physiological principles discovered during its research collaboration with NASA to develop another promising technology: an intrathoracic pressure regulator for patients requiring ventilation assistance because they are too sick to breathe on their own.
ResQPOD® is a registered trademark of Advanced Circulatory Systems Inc.

The ResQPOD is an impedance threshold device used to enhance circulation during CPR. It could be used to increase circulation for astronauts as their bodies initially adjust to a return to gravity from the weightlessness of space.
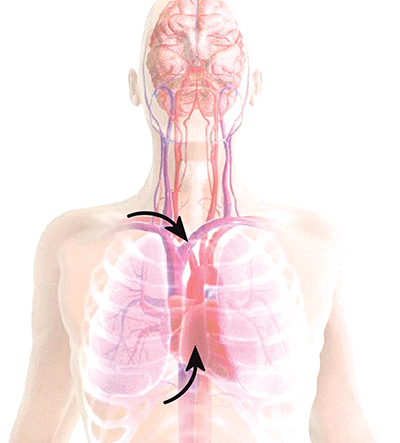
CPR delivers approximately 15 percent of normal blood flow to the heart.
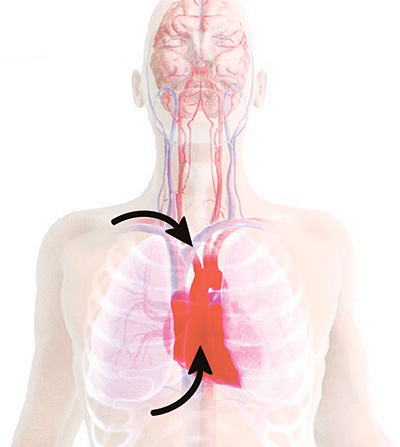
The ResQPOD doubles blood flow back to the heart.
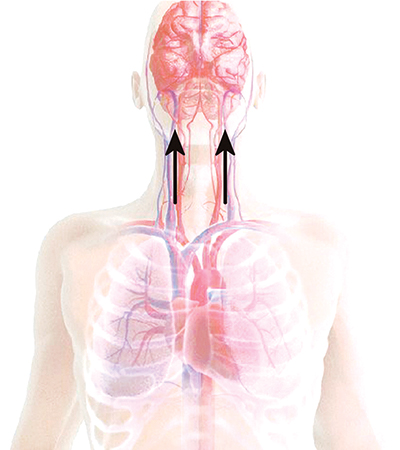
CPR delivers approximately 25 percent of normal blood flow to the brain.
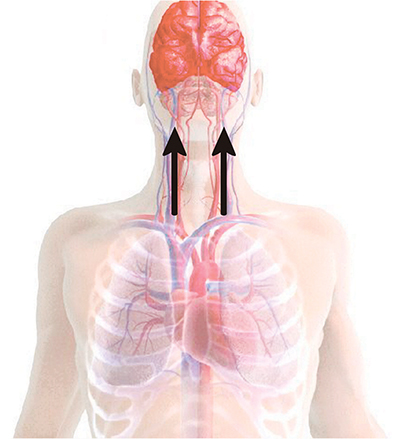
The ResQPOD delivers more than 70 percent of normal blood flow to the brain.

The ResQPOD increases circulation in states of low blood pressure. When used on patients in cardiac arrest, the ResQPOD harnesses the chest wall recoil after each compression to generate a small but critical vacuum within the chest. This vacuum enhances blood fow back to the heart and results in a marked increase in blood fow out of the heart with each subsequent chest compression.