Catalytic Converters Maintain Air Quality in Mines
NASA Technology
In the 1980s, Langley Research Center teamed up with the Federal Aviation Administration to develop a technology for the space-based detection of wind shears, which are rapidly changing wind currents occurring during thunderstorms. Downbursts in particular can wreak havoc on airplanes during take-off and descent and have caused several aircraft crashes over the years. One approach NASA tried was based on Lidar technology, which uses pulsed lasers to measure the distances and shapes of objects.
Carbon dioxide (CO²) lasers were an ideal tool for the job because of their energy efficiency, stability, and relative safety. But during operation, the CO² is broken down by the laser into carbon monoxide (CO) and oxygen, leading both to CO² depletion and oxygen build-up that can eventually oxidize electrodes, causing arcing and power degradation, which can have disastrous effects on the optics.
To prevent this from happening, the laser can be periodically flushed out, or run “open” with a constant feed of fresh laser gas mix while bleeding spent gas, neither of which are viable options in space-based applications. So NASA conceived of another, more convenient method: a tin oxide-based catalyst applied to a washcoat, which is then deposited with platinum clusters. While the tin oxide gathers oxygen, CO attaches to the platinum and serves as a catalyst, causing the oxygen and CO to combine to form CO². In one fell swoop, CO² is conserved and the threat of signal degradation, arcing, optics damage, and the necessity of multiple tanks of make-up gas is averted.
This conversion process is similar to how a standard automotive catalytic converter would function, but with a key difference. A gasoline-powered automobile’s converter functions best at high temperatures, or when the engine is operating at or near full capacity. It doesn’t work well in the minutes after a car is started or when it’s running idle. NASA’s converter, by contrast, functions at low temperatures due to the special mixture of chemicals contained in the washcoat.
Technology Transfer
Zaki Mustafa had worked many years as an executive for Corning Inc., a company that manufactures substrates for catalytic converters. He heard about the NASA-developed catalyst from friends working at Rochester Gas & Electric, which was licensing the technology for another application. After looking into it, Mustafa realized the technology’s advantage: Its low-temperature capability meant it had the potential to function right from the moment a vehicle was started all the way to when it was switched off. There wouldn’t be a downtime in performance that allowed for toxic gases to escape into the air. “I had a gut feeling that this technology had a lot to offer,” he says, “so I went for it.”
Soon after he helped found Airflow Catalyst Systems Inc. in Rochester, New York, in 1996. The company licensed the tin oxide-based catalyst technology from Langley in 1997 and started working in collaboration with NASA to adapt the technology for commercialization.
Changes were made to prime the technology for use in exhaust systems. Unlike the CO² laser NASA contended with, exhaust systems, in addition to CO, also release hydrocarbons from evaporated, unburned fuel, as well as oxides of nitrogen, which contribute to smog and acid rain. For that reason, automobiles typically have what are called three-way catalytic converters. The tin oxide-based catalyst was transformed into a three-way converter, first by adding certain rare metals to the washcoat, which catalyzed the reduction of nitrogen oxide into nitrogen and oxygen. As for the hydrocarbon, because it undergoes the same oxidation as CO and is converted to CO² and water, only minor tweaking was needed to the existing technology.
Following these improvements, NASA and the company worked together to stabilize the converter’s performance at higher temperatures; the special washcoat that had allowed it to function at low temperatures didn’t hold up as well when exposed to the piping hot conditions of full-capacity internal combustion engines. By adding various lanthanide metals, says Langley engineer Neal Watkins, “We managed to keep it working for 25,000 miles, which is on the order of where you need to be for an after-market catalytic converter.”
In 2004, Mustafa reached a fork in the road: He could either attempt to enter the automotive market, which was saturated with large, established companies, or he could offer a new product for an industry ripe for innovation. He decided on the second approach and aimed to introduce the technology for use in off-highway diesel equipment. The goal was to replace high-maintenance archaic active paper filter devices with fully regenerative passive systems requiring practically no maintenance.
In 2010, after years of additional development, Airflow Catalyst Systems began selling its EZ-series passive filters—available in the EZCat, MinNoCat, and EZDOC models—for diesel-operated machinery, namely those used in the mining industry.
Benefits
The EZ-series filter is an entirely new technology for the mining sector, where safety regulations have become more stringent in recent years in recognition of the dangers in that environment. For example, the buildup of CO—a colorless, odorless, and tasteless gas that is toxic to humans—can be deadly and tends to happen especially in the wake of a disaster. The farther down a mine one goes, the more dangerous such a threat becomes.
“The deeper you go underground, the more difficult it is to circulate ventilation air through the mine,” says Gary Robb, the company’s director of development. “As much as possible, you need to reduce any form of pollutant directly at the source.”
That’s where the NASA-derived three-way washcoat comes into play. It neutralizes CO, hydrocarbons, oxides of nitrogen, and particulate matter emissions (the toxic, black smoke that puffs out of trucks) from any diesel-operated equipment, such as scoops and roof bolters. EZ-series filters remove more of these toxins than the mechanical filters they replace, and they require much less maintenance.
“Those mechanical filters, which only remove particulate matter, may have to be replaced during the course of a work day,” Mustafa says. “That can be costly and labor intensive.” But because the EZ-series converts particulate matter into harmless gases, there is no buildup of residue, and therefore no filter replacements are needed. Says Mustafa, “The operator should never have to touch our system on a daily basis.” In fact, EZ-series converters are projected to last for at least 7,000 hours, with appropriate duty cycles. The first products, which were sold in 2010, are still functioning and have yet to accumulate that much operating time.
Since the company started selling the filters, the response from industry has been strong, with business picking up every year. “Our reputation with customers is solid,” says Umbereen Mustafa, the company’s business manager, “and it’s not just from a product standpoint, but also in terms of our customer service.”
In addition, the Mine Safety and Health Administration, the federal agency that oversees mining regulations, commended Airflow Catalyst Systems for introducing to the industry the first substantial technology advancement of its kind in the last two decades.
For Mustafa, the long research and development phase that paved the way for the EZ-series was worth the wait. “With this NASA technology, we developed a product and we’ve found ourselves a niche that has set us up for success,” he says.
EZCat™ MinNoCat™, and EZDOC™ are trademarks of Airflow Catalyst Systems Inc.

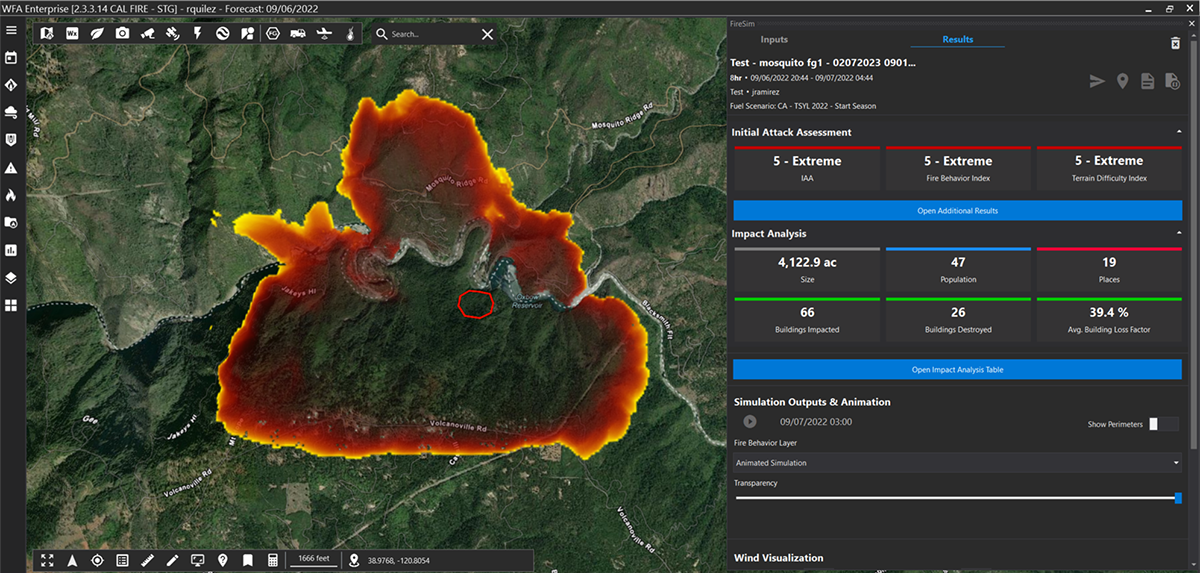
Airflow Catalyst Systems’ EZ-series of converters, derived from technology developed and licensed by NASA, are used in the exhaust systems of underground diesel mining equipment to purge the air of potentially dangerous fumes. An EZ converter is installed for such an application.
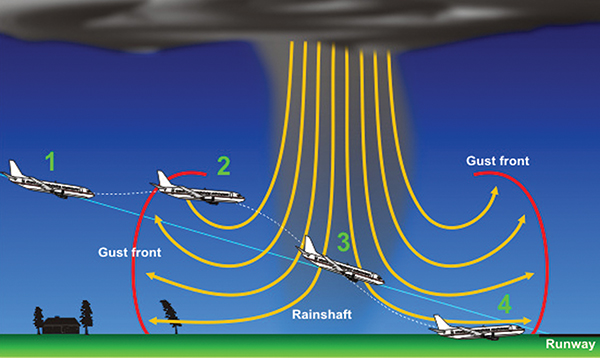
NASA teamed up with the Federal Aviation Administration in utilizing carbon dioxide lasers for Lidar technology, which planes could use to account for dangerous downbursts of wind that can occur during storms.