A Head Start to a Healthy Heart
Sudden cardiac death (SCD), or cardiac arrest, is a leading cause of death among Americans. The American Heart Association estimates that SCD kills over 300,000 adults in United States each year, 95 percent of whom die before they reach a hospital or another source of emergency aid, such as defibrillation.
Often mistaken as a heart attack, SCD is commonly triggered by an arrhythmic disorder called ventricular fibrillation, which disturbs the ventricles' blood-pumping action and causes them to contract in an irregular fashion. Sudden death can also result from ventricular tachycardia, which causes the ventricles to beat too fast, ultimately affecting their ability to pump blood; ventricular tachycardia may develop into ventricular fibrillation. As serious and as fatal as SCD is, cardiologists for years have lacked the proper diagnostic tools needed to gauge risk factors so that protective measures can be offered.
In response to the demanding need for an enhanced assessment test—and in order to offer reassurance to cardiologists and patients alike—Bedford, Massachusetts-based Cambridge Heart, Inc., has licensed the only U.S. Food and Drug Administration (FDA)-cleared tool to identify those at risk for SCD. The Microvolt T-Wave Alternans Test™ was invented by Dr. Richard J. Cohen, a professor at the Harvard-Massachusetts Institute of Technology (MIT) Division of Health Sciences and Technology, with developmental support and funding from NASA's Johnson Space Center and the National Space Biomedical Research Institute (NSBRI) in Houston, Texas. In 1993, MIT licensed the technology to Cambridge Heart, Inc., a start-up company that Dr. Cohen helped to establish.
Cambridge Heart's noninvasive technology measures T-wave alternans, a change from one heartbeat to the next that is too minute to be detected by a standard electrocardiogram. Cardiac patients with such a change in heartbeat regulation are faced with a much greater risk of ventricular arrhythmia and SCD than those without it. The company's ability to measure electrical alternans on a microvolt level has been clinically proven to be just as accurate as—and in some studies, more accurate than—more costly and somewhat risky, invasive procedures, such as electrophysiological testing that involves the insertion of a catheter into a patient's heart to deliberately induce ventricular arrhythmias.
While other noninvasive measurement tests are performed during rest or normal activity the Microvolt T-Wave Alternans Test is administered while patients' heart rates are elevated, during exercise or pharmacological stress. Clinical studies have demonstrated that elevating the heart rate increases the sensitivity of Microvolt T-Wave Alternans testing in identifying patients at risk of SCD.
The predictive accuracy of Cambridge Heart's Microvolt T-Wave Alternans Test spawns from the company's Analytic Spectral Method,TM which determines precise microvolt measurements well below normal noise levels, and its unique, Micro-V Alternans Sensors, high-resolution electrodes that allow for the analysis to be performed in conjunction with noisy exercise activity, such as treadmill or bicycle use.
The Microvolt T-Wave Alternans Test is processed through Cambridge Heart's HearTwaveTM System, which is compatible with virtually all existing stress systems, electrophysiology monitoring devices, or electrocardiogram carts. The HearTwave simply collects and computes the alternans data, and then automatically prints out a real-time report.
The test has proved successful in multiple clinical trials conducted around the world in a wide variety of patient populations, including those who have experienced heart failure or myocardial infarction. It can also help physicians manage the most difficult cases, such as the analyses of young patients who appear to be in good health. For example, in a clinical study of 130 syncope patients (those who experience temporary impairment of blood circulation to a part of the body, which often leads to loss of consciousness), the Microvolt T-Wave Alternans Test predicted SCD-related events with a high relative-risk rate, whereas electrophysiologic testing of these patients failed to achieve statistical significance as a predictor of such events.
Cambridge Heart's original stress testing machine, the CH2000, served as the platform for the Microvolt T-Wave Alternans Test. The company advanced the original CH2000 model to detect T-wave alternans, and teamed up with Netherlands-based Philips Medical Systems International to market the technology exclusively throughout the United States and Canada.
Additionally, Spacelabs Medical, Inc., a leading provider of integrated healthcare information systems and instrumentation, was granted clearance from the FDA to incorporate Cambridge Heart's Microvolt T-Wave Alternans Test within its Burdick® Quest® exercise stress system. Used in conjunction with the test, the Burdick Quest system, which combines innovative touchscreen technology with leading-edge diagnostic capabilities, will further help cardiologists in their efforts to predict the risk of life-threatening arrhythmias associated with SCD.
NASA and the NSBRI are currently supporting research involving the Microvolt T-Wave Alternans Test to determine whether space flight increases the risk of severe ventricular arrhythmias, and, if so, whether effective countermeasures can be developed.
Microvolt T-Wave Alternans Test,™ Analytic Spectral Method,™ Micro-V Alternans,™ and HearTwave™ are trademarks of Cambridge Heart, Inc.
Burdick® and Quest® are registered trademarks of Spacelabs Medical, Inc.
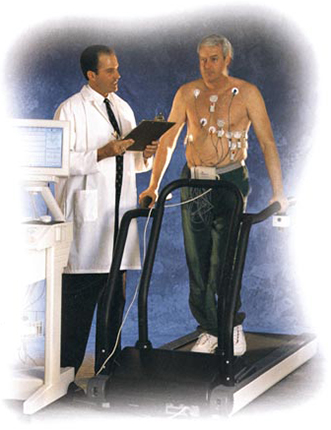
Unlike other noninvasive measurement technologies performed during rest or normal activity, the Microvolt T-Wave Alternans Test™ is conducted while the heart rate is elevated, generally during exercise or pharmacological stress.

The HearTwave™ System computes microvolt T-wave alternans and provides trend reports with guided interpretation for quick, accurate analysis.