NASA’s VITAL Contribution to Global Pandemic Relief
Subheadline
NASA-designed ventilator helps save lives in developing nations
In 2020, the COVID-19 pandemic forced institutions to lock down, and patients poured into hospitals around the world. But in both the United States and developing nations, one previously little-used piece of medical equipment was suddenly swamped by demand: the ventilator.
Ventilators are used in hospitals to assist patients with breathing, acting as an external set of lungs to push air down the windpipe and aid the respiratory system. While these machines can save lives, hospitals typically only have a few on-site, and they can only be operated by professionals with specialized training. With a respiratory illness like COVID-19 inundating intensive care units with patients in need of breathing assistance, ventilators were in short supply.
In parts of the world with fewer hospitals and less healthcare infrastructure, the situation was even more dire. In a country like India with a billion people, many of them in rural regions, the ventilator supply was impossible to triage. And 9,000 miles away in Brazil, the disease spread rapidly, infecting millions in the first year.
With such a desperate need for these specialized machines, the engineers at NASA’s Jet Propulsion Laboratory in Southern California figured they could use their experience with solving problems and building cutting-edge technology.
“For us, one of the hardest things is to be told that the best thing you can do to help is to just go home. We’re engineers. We like to fix things,” said Stacey Boland, an engineer at JPL who was part of the team that worked on the ventilator. “And so that kicked off a conversation, and in pretty short order we started brainstorming.”
By removing features that weren’t necessary for most cases and keeping the design and operating interface simple, the team was able to quickly assemble a ventilator – one that could be manufactured with “off-the-shelf” parts that aren’t used in standard ventilators, eliminating competition for supplies. In less than one month, JPL built a prototype of a working ventilator, dubbed VITAL (Ventilator Intervention Technology Accessible Locally). Soon after, the Food and Drug Administration (FDA) gave emergency authorization to use the device in hospitals. Through the center’s Technology Transfer Office, JPL made a cost-free, non-exclusive license available for companies to produce their own versions of VITAL.
“We had a lot of discussions early on about what was the right thing to do, how could we actually get this design out in the world as quickly and effectively as possible,” Boland said. “We wanted to make sure that if ventilators were needed anywhere in the world, this technology would be something that could be used to serve that need.”
Ensuring Vitality
Over 100 companies applied for the license globally, and 31 were awarded a license to produce the commercial versions of VITAL. STARK Industries LLC of Columbus, Ohio, was one of eight U.S. companies. As a medical technology firm, STARK had taken a keen interest in VITAL as soon as the company’s leadership heard about it.
STARK was founded after Joe Swantack had an idea to build a resistive exercise device. When looking for a heart-rate sensor to pair with the device, Swantack found Dr. Peter Lee, a cardiothoracic surgeon, and together they discovered a wireless cardiac monitor patch. The two joined forces, and in 2016, STARK Industries was formed.
Because his device, which builds muscle without weights, had clear benefits for astronauts’ exercise needs in zero gravity, Swantack has since worked with NASA personnel several times, and when the coronavirus pandemic began to take hold, the company leadership learned about JPL’s invention.
After seeing how useful the machine would be for struggling hospitals, STARK created a subsidiary spinoff called Spiritus Medical Inc. in December 2020, completely devoted to the production and sale of VITAL. Lee said that in the United States sales of the Spiritus ventilator, now renamed the Vitality ventilator, are intended for use as a “stockpile ventilator,” to be used in situations when there is an unexpected surge in the need for ventilators that greatly exceeds hospital capacity at the time.
“To use a car analogy, most U.S. hospitals have Cadillacs in their ICUs. This ventilator is a basic Ford. It gets you from point A to point B and doesn’t have all the bells and whistles,” said Lee. “This can take care of 80% to 90% of patients who need to go on a ventilator, so those higher-end ones can be reserved for those who absolutely need them.”
Spiritus has manufactured and sold over one hundred ventilators abroad and continues to work with international partners to bring its version of VITAL to countries around the world. The company is actively working with partners in several developing countries such as Nigeria and Ghana. Additionally, Spiritus has forged a strong relationship with companies like Bharat Forge Ltd. in Pune, India. As one of the most populous countries in the world, India was heavily affected by the COVID-19 pandemic and suffered from a lack of readily available ventilators. Because of this, several international partners in VITAL are from the South Asian nation.
Forging a New Path
Bharat Forge is the flagship company of the Kalyani Group, a multinational conglomerate whose primary business is in forging and manufacturing metal components, from car parts to military machinery. When India needed ventilators in more rural areas, the Kalyani Center for Technology and Innovation decided to develop and produce one. Its original design worked but would’ve required additional approvals for international export. As VITAL already had approval from the FDA in the United States, it was a good match for the company’s production goals.
Bharat Forge applied for a license from JPL and soon became one of the international licensees. Jagdish Sherkar, senior manager of the Kalyani Center, said the company’s manufacturing and distribution agreement with Spiritus Medical comes from a mutual need to keep the VITAL supply chain going.
“We joined hands to serve a greater population residing in Asia, the Middle East, and Africa,” said Sherkar. “We had manufacturing strengths, Spiritus has a global reach. During lockdown periods, if one country is shut down, the other could supply the needy.”
CuraSigna Systems Pvt. Ltd. in Bangalore, India, also said the country’s struggles with a supply of ventilators was its impetus for licensing VITAL. Founded in 2020 during the earliest stages of the global pandemic, the company’s founders saw the surges of cases paired with a lack of resources and searched for ways to help.
Srishti Ramasubramaniam and Rama Subramaniam, cofounders of CuraSigna through the Sushil Swabhiman Private Trust, saw that VITAL was licensable, and with the additional help of the Indian Space Research Organization, the company was able to build a new version of VITAL. CuraSigna is integrating its version of VITAL with an entire ecosystem of medical technologies. Dubbed “ICU-on-Cloud,” CuraSigna’s medical technology is tightly integrated with apps built on the Android mobile operating system. Other devices built on this platform include, but aren’t limited to, electrocardiogram heart monitors and intravenous infusion pumps.
“The whole objective is to take the best intensive care doctor to the last patient. Unlike most ventilators, we can take it literally anywhere,” said Subramaniam. “And doctors, instead of doing physical rounds, they can use a Samsung Galaxy Fold to do virtual rounds.”
CuraSigna is developing other technologies using processes derived from NASA research like air purification systems that use photocatalytic oxidation. Subramaniam believes both innovations developed at NASA and the way the company’s core team worked closely with NASA engineers were instrumental in his company’s success.
“We’ve saved tens of thousands of lives and will probably go on to save more.” Subramaniam said. “We can’t thank NASA enough.”
Saving Lives from São Paulo
Brazil was another country hit hard by the pandemic, with millions of cases reported in the first year. To Juan Ruben Calbucoy OIiarte, watching the news was terrifying.
“The most dramatic moment was to see images of doctors using plastic bags as a last resort in a desperate attempt to save the lives of their patients,” said Oliarte.
As the founder and CEO of Russer, a medical equipment manufacturer based in the city of Indaiatuba in São Paulo state, Oliarte wanted desperately to do something. But his usual business was in urology supplies, not pulmonary care. Russer couldn’t produce something on its own.
“Providentially, leafing through the newspaper I found an article reporting that NASA JPL offered the patent and license to manufacture a dedicated pulmonary ventilator for COVID,” Oliarte said. “I didn’t hesitate for a moment and applied to receive such a valuable contribution.”
Russer now produces a version of the ventilator called VIDA or VP-VITAL. Brazil's infrastructure presents challenges for building equipment like VITAL. The country has a different electrical system than the United States and rural parts of the nation might not maintain a surplus of ventilator equipment. In order to adapt the machine, the company brought it into compliance with local standards set by the Brazilian Ministry of Health and worked to ensure VITAL functioned all over the country. Russer has delivered 300 ventilators to hospitals in several regions around Brazil, both in remote places as well as some of the country’s larger urban areas.
The JPL team still stays in touch with licensees, answering questions or giving technical advice on how to improve designs. Boland believes worldwide collaborations like this were unprecedented for the center.
“We’re used to licensing technology to one or two companies, not 20-something,” Boland said. “And the level of effort it took and the amount of support we were able to provide, it was really a privilege to get to work on something like this. I think it’s been a very positive experience for all involved.”

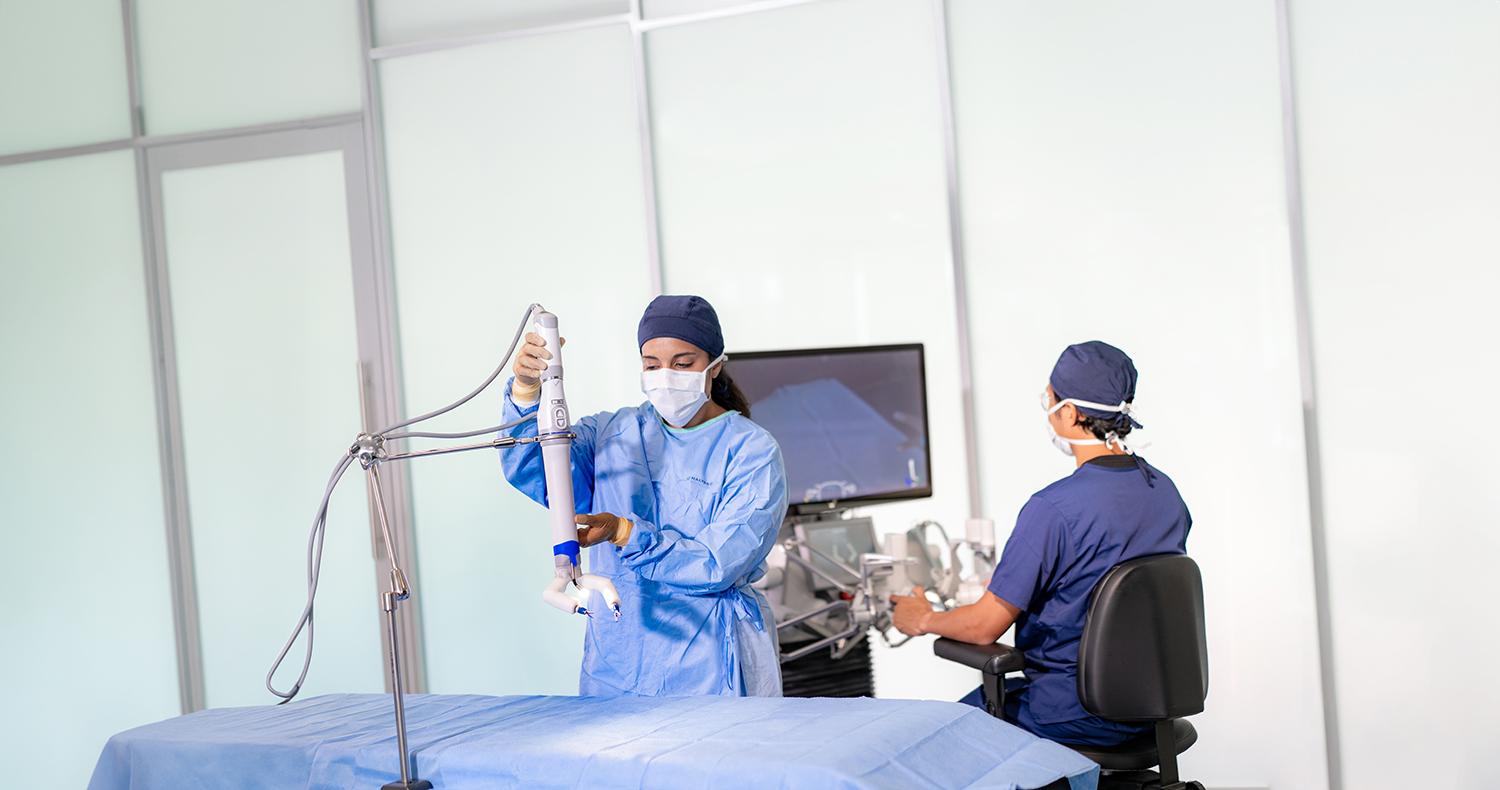
Engineers at NASA's Jet Propulsion Laboratory in Southern California prepare to ship a prototype ventilator for coronavirus patients to the Icahn School of Medicine at Mount Sinai in New York. Credit: NASA

To make sure ventilators could be quickly manufactured and administered to those in need during the COVID-19 pandemic, a team of engineers at JPL created the Ventilator Intervention Technology Accessible Locally (VITAL) device, made of off-the-shelf parts. Credit: NASA

Joe Swantack poses with a VITAL ventilator. His company, STARK Industries, was one of the first companies to receive a license from JPL to build a commercial version of VITAL. Credit: Spiritus Medical Inc.
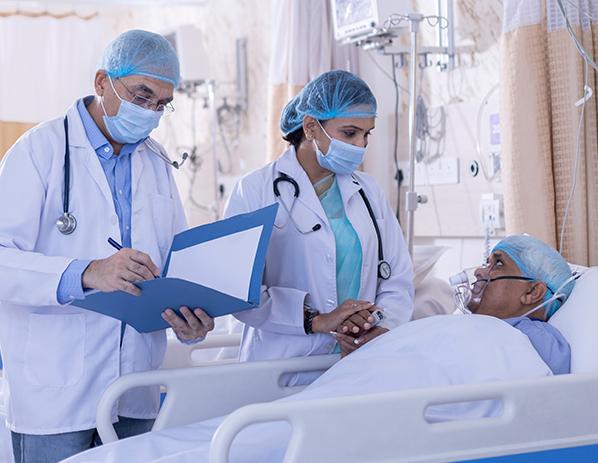
In countries like India, the pandemic caused a shortage of ventilators. Under license from NASA, companies like Bharat Forge built versions of the VITAL ventilator to help treat patients at hospitals in need of equipment. Credit: Getty Images

The CuraSigna implementation of VITAL is integrated with a service called ICU-on-Cloud, allowing intensive care doctors to keep tabs on all their patients from a tablet. Credit: CuraSigna Systems Pvt. Ltd.

While Russer’s implementation of VITAL may look similar, it’s had changes to adapt and bring it into standards for use in Brazil, where medical infrastructure can be inconsistent. Credit: Russer Brasil