Photocatalytic Water Splitter Stores Energy as Hydrogen
NASA Technology
At a glance, the surface of Mars appears to offer little in the way of natural resources. Scenes captured by the various rovers exploring the planet depict a desolate, dusty landscape, messily strewn with rocks and punctuated by occasional craggy outcrops, stretching to meet the rusty sky in all directions.
Researchers are still working to determine the composition of the Red Planet’s geology, but analyses by NASA’s rovers in recent years have confirmed the long-suspected presence of significant amounts of water—one of any Earthling’s most precious resources—frozen in its surface. The find was significant to those planning missions to Mars, not just because of water’s inherent life-giving properties, but also because of its constituent parts: oxygen, that other most precious resource to Earth fauna, and hydrogen, NASA’s rocket fuel of choice.
In anticipation of interplanetary missions, NASA has long worked to find the most efficient ways to separate and recombine molecules such as water in order to make the most of whatever scant resources may be available on an extraterrestrial body. Such technology also has other space applications. For example, oxygen generators aboard the International Space Station create breathable air by separating oxygen from water using an electric current, a process called electrolysis. This method can also be used to purify water.
When John Guerra, president and CEO of Cocard, Massachusetts-based Nanoptek Corporation, and his team set about creating a semiconductor that could use sunlight to harvest hydrogen from water, though, they were also thinking of earthbound applications, like storing energy for backup power and running hydrogen fuel cell vehicles.
Japanese researchers had discovered decades earlier that titanium dioxide, or titania, could function as a photocatalyst to split water molecules when illuminated with ultraviolet (UV) light. However, Guerra says, “The limitation that it only responded to UV light made it inherently inefficient in sunlight.” Only about 4 percent of the photons in sunlight have wavelengths in the UV range or shorter, while the sun’s strongest output is in the lower-frequency visible range. Ultraviolet light also takes more energy to produce artificially than visible light.
Scientists had worked to push titania and other semiconductors to respond to visible light, but their techniques, such as doping the substances with ions, tended to make them less stable such that they would break down in an electrolyte solution. Water in this application is typically combined with an electrolyte to make it conductive.
Shortly before applying for their first NASA Small Business Innovation Research (SBIR) contract in 2002, Nanoptek’s scientists discovered a way to push titania’s responsiveness into the realm of visible light without altering its stability. Instead of ion doping, they stretched the material’s molecules.
Making titanium dioxide an electrically active semiconductor when exposed to visible light meant shifting its bandgap—the difference in energy between the valence and conduction bands in the semiconductor. In its natural state, titania responds to electromagnetic wavelengths of around 400 nanometers or less, frequencies beginning at the upper end of the visible spectrum. Only these relatively high-frequency, high-energy photons deliver enough energy to cause its electrons to jump from the valence band to the conduction band, creating a charge pair. But the visible light that constitutes the sun’s most abundant output has wavelengths between 400 and 780 nanometers, meaning most of it is lost on titania.
That the properties of a semiconductor can be altered by tensile stress was already known to industry, largely as a problem, but Nanoptek’s scientists recognized this “weakness” as a potential tool to engineer the bandgap. The problem was maintaining a high enough level of stress without the substance bursting apart. The company’s solution was to grow the titania crystals on a surface that was nanostructured with tiny peaks. As each successive layer of the crystalline lattice is added, the strain on its surface increases. “It’s going to grow in such a way that it stretches to line up with the lattice below it,” Guerra explains. At the same time, the nanostructures provide additional surface for the titania to adhere to.
“If you tried it on a flat surface, you could only get up to a few megapascals of strain before the whole thing explodes off the surface,” he says. “Now we can develop extremely high strain, into the gigapascal range.” A gigapascal translates to a force of about 145,000 pounds per square inch. It took years to get from the company’s discovery to the development of a durable surface that could maintain this kind of pressure and could be mass- produced economically, but the work began in earnest with the company’s Phase I and II SBIR contracts with NASA’s Ames Research Center.
“For Nanoptek, that funding was incredibly important, not only in economic terms but also from the standpoint of validation,” Guerra says. “When you’re at that stage, you’re not going to find private funding. The SBIRs allowed us to hire people, expand our lab, and explore the parameters of what we had discovered.”
Technology Transfer
The contract also helped keep the company focused on developing a product that not only worked but was commercially viable, bearing in mind factors like cost and marketability, Guerra says. “That was our goal anyway, but it was very helpful to have the funding entity have that point of view.”
During its SBIR work, the company molded polycarbonate discs with nanostructured surfaces and then applied titanium dioxide, a process that allowed it to explore nanostructures of different sizes and shapes and to verify the effectiveness of the strained semiconductor surface. The work succeeded in lowering the titania’s bandgap to the point that it was activated by light at frequencies of 529 nanometers and higher, well into the visible spectrum. It now made use of 29 percent of the sun’s light, as opposed to the 4 percent that activates ordinary titanium. But the surfaces only lasted about half an hour in the solution of water and hot electrolytes.
Following the NASA work, Nanoptek went on to win funding from the Department of Energy and the state of Massachusetts and then subsequently from private investors, allowing the development of a less expensive, more efficient, and more durable product. Now the nanostructures are etched cheaply into less-costly commercial-grade titanium, and the titanium dioxide is formed through thermal oxidation, which is also inexpensive. More importantly, thermal oxidation causes the titania crystals to grow down into the metal, as well as upward. “You’re sending roots into the substrate, as well as leaves into the electrolyte,” Guerra says.
The result is a surface so durable the only way to remove it is through grinding.
Nanoptek’s scientists also found that straining the semiconductor’s molecules not only lowered its bandgap but had the unexpected effect of increasing the mobility of positive charges in the material, which allows more time for the positive and negative charges activated by sunlight to do the work of splitting water atoms rather than recombining prematurely. Also serendipitously, it brought the electron voltage of the material’s conduction band above the hydrogen evolution potential—the voltage required to cause hydrogen to separate from water molecules spontaneously. This makes hydrogen production more efficient, causing it to occur in sunlight with no added electrical charge.
“But we choose to add electricity because it’s more important to make a lot of hydrogen cheaply,” Guerra says.
To split water simply by running a charge through it requires two to three volts, depending on the electrolyte and properties of the electrodes, Guerra explains. But the hydrogen harvested in this way would only generate something like one-half to 75 percent of that energy. When illuminated, Nanoptek’s titania photoanode requires less than one volt to produce an equivalent amount of hydrogen, and so is two to three times more efficient on an electrical energy basis. To maximize return on capital, though, Nanoptek’s go-to-market model uses both a titania photoanode and a conventional anode, which operates on 1.8 volts at 82 percent efficiency. The two anodes can be run during sunlight simultaneously to produce a blended efficiency of 97 percent, and the conventional anode can continue to produce hydrogen through the night with low-cost, nighttime grid electricity or with wind electricity, which is typically stronger at night.
Benefits
For any grid based on intermittent power sources—which describes most renewable energy sources, such as wind and solar power—the ability to store excess energy for times when there is no wind or sun is crucial. Nanoptek’s technology efficiently converts excess power at any time of the day into hydrogen and stores it. “Then, by using a fuel cell, you can convert that hydrogen back to electricity in an on-demand way,” Guerra says.
It’s the first electrolyzer to be competitive with batteries and even provides some advantages. For example, unused alkaline batteries lose energy over time, whereas a cylinder of pressurized hydrogen gas will keep virtually indefinitely without losses. Batteries also lose energy to heat if power is drawn from them rapidly, which is not the case with hydrogen fuel cells. At telecommunication towers in remote parts of Africa or India, which are often powered by solar energy, batteries have performance issues due to the heat. “Our panels love heat,” Guerra says. “The hotter the electrolyte, the more efficient they get.”
Nanoptek envisions power plants for communication towers in the developing world scaled to also power microgrids for local communities, using the company’s technology to maintain a constant energy supply. “It’s a way to leapfrog an unreliable grid and deliver reliable, low-cost power to people in those parts of the world,” he says. “The R&D part for us is largely done, and the challenge now is to find funding to scale up manufacturing so the technology can be deployed and start positively impacting people’s lives.”
By the end of 2014, the company was taking its first orders and gearing up for production. For now, the technology is being marketed to businesses for large-scale operations. Companies whose trucks or forklifts run on hydrogen fuel cells generally have plenty of roof space for a set of Nanoptek’s photocatalytic panels to power their fleets, Guerra notes, adding, “There are also a lot of companies out there that use hydrogen in their manufacturing processes, and a lot of them are interested in having a cost-efficient way to make the hydrogen on site.”
The number of panels can be scaled up or down according to needs.
He points out that automakers and governments are beginning to turn their attention to hydrogen fuel cell vehicles, with car companies beginning to unveil their first hydrogen-powered production models and California building hydrogen filling stations. He hopes Nanoptek’s technology will prove integral to the new, hydrogen-based transportation infrastructure. “Our success depends on the success of other companies, but their success depends on a low-cost source of hydrogen, and that’s where we come in,” he says.
The systems are not yet economical or safe for residential use, as the compressor and dispenser required forrefueling are still too expensive for an individual homeowner, but Nanoptek envisions the first residential use being in planned developments where a cluster of homes could be outfitted with panels. Homeowners could chip in for a shared compressor and dispenser, using the system to power hydrogen cars.
Guerra also predicts the technology will one day circle back to the Space Agency that first funded it, where it will be used for planetary exploration. “Our devices, with sunlight and local water on an extraterrestrial body, will be used to make hydrogen fuel for rockets and oxygen for habitats,” he says. In fact, for each kilogram of hydrogen the system produces, it can make about eight kilograms of oxygen.
“It was really the NASA funding that financially got us off the ground and also established the validity of the technology,” he says. Whether that technology will one day get NASA rockets off the ground of extraterrestrial bodies remains to be seen.

As automakers and governments begin to turn their attention to hydrogen fuel cell-powered vehicles, Nanoptek hopes its technology will become integral to the new, hydrogen-based transportation infrastructure. Image courtesy of the U.S. Navy
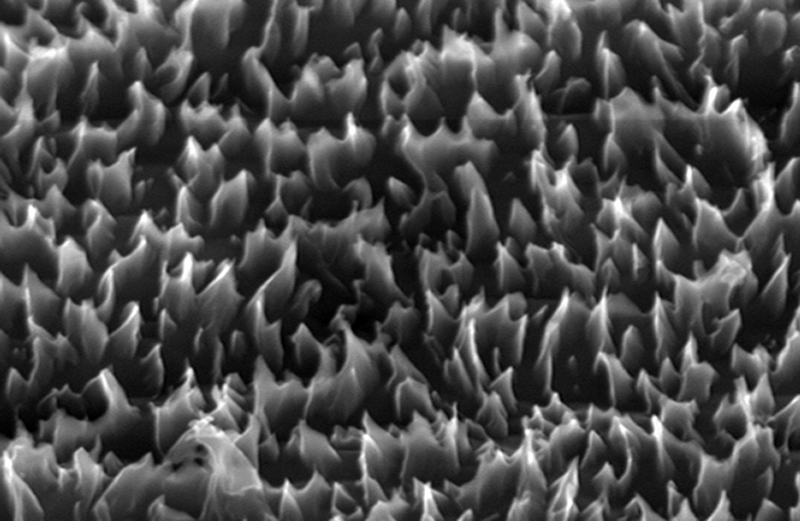
By growing titania crystals on a surface nanostructured with tiny peaks, Nanoptek is able to strain the crystals’ surfaces, shifting this semiconductor’s bandgap—the difference in energy between its valence and conduction bands—to make it responsive to more of the solar spectrum.

Nanoptek designed this small electrolyzing panel for use in gas chromatography in the field.

In hotter parts of the world, the batteries used to store energy from solar panels often have heat-related performance issues that a tank of hydrogen stored for energy would not. What’s more, Nanoptek’s photocatalytic panels become more efficient the hotter they get. The company envisions solar power plants for communication towers in the developing world scaled to also provide reliable power to microgrids for local communities.

With the long-term goal of sending astronauts to Mars, NASA has an interest in any technology that might be able to use sunlight to split water molecules from the Martian soil into oxygen for life support and hydrogen for rocket fuel.